基于知识驱动的解释性条件扩散模型用于无对比剂心肌梗死增强合成|文献速递-医学影像算法文献分享
Title
题目
Knowledge-driven interpretative conditional diffusion model forcontrast-free myocardial infarction enhancement synthesis
基于知识驱动的解释性条件扩散模型用于无对比剂心肌梗死增强合成
01
文献速递介绍
无对比剂人工智能心肌梗死增强(MIE)合成技术(如图1所示)仍是一个具有挑战性但临床意义重大的问题。主要挑战在于两个方面:(1)在不使用对比剂(CAs)的情况下,心肌瘢痕在CINE序列上通常不会表现出明显的对比增强,甚至可能不可见(Ordovas和Higgins,2011),这导致准确合成缺乏可靠的视觉参考;(2)心肌梗死患者在心脏解剖结构和瘢痕分布方面存在显著的个体间差异(Richardson等,2015),这对瘢痕的精确定位构成了重大挑战。这些问题的复杂相互作用使得传统的纯数据驱动方法(Xu等,2020;Zhang等,2022)难以在不同个体间有效泛化。这些方法(图2(a))仅仅在非增强图像输入(CINE序列和T1序列)与增强图像输出(LGE图像)之间建立了简单的直接映射(Xu等,2020;Zhang等,2022),缺乏对输入和输出之间复杂关系的理解。 近年来,知识与数据驱动方法(如图2(b)所示)(Qi等,2024)在应对MIE图像合成挑战方面展现出巨大潜力。该方法首次引入与心脏运动学相关的心脏力学知识,以指导心肌运动模式的学习,而不是仅仅依赖从非增强图像到增强图像的直接数据驱动映射。通过分析异常的功能性运动模式,如局部增厚减少或运动不同步,我们的模型即使在心肌瘢痕不直接可见的情况下,也能推断出其存在。这种方法利用了心肌运动模式与潜在心脏异常之间的关系,使模型能够将功能动态映射到心肌异常并推断心肌梗死区域。此外,整合从T1序列(包括8幅原始反转恢复加权(IRW)序列图像)中提取的形态学信息,可提供丰富的结构背景。运动学和形态学特征的融合形成了对心脏更全面的表征,使模型能够适应不同患者间观察到的显著个体解剖差异。与传统的纯数据驱动直接映射方法(如图2(a)所示)(Xu等,2020;Zhang等,2022)相比,心脏力学知识与图像数据(CINE和T1序列)的结合不仅帮助模型理解如何学习这些特征,还有助于理解数据映射的基本原理,从而指导模型如何以及为何合成MIE图像。这种知识与数据驱动的方法为克服心肌瘢痕的细微不可见性和患者间高个体差异所带来的固有挑战奠定了更坚实的基础。 然而,这种目前唯一的知识与数据驱动方法(Qi等,2024)在运动学推理的可解释性、形态学知识整合以及运动学-形态学融合方面仍存在局限性,如图3所示。运动学推理的可解释性局限:Qi等(2024)采用了一种隐式推理策略,将异常心脏运动与心肌瘢痕广泛关联,而没有明确建模运动异常与瘢痕形成之间的因果关系。这种缺乏明确因果建模的情况导致决策过程不透明,使得临床医生难以理解和信任模型的预测,特别是在推断不可见的心肌瘢痕时。形态学知识整合的局限:Qi等(2024)使用多通道输入编码器以纯数据驱动的方式融合T1序列图像来提取形态学特征。这种策略未能纳入特定领域的形态学知识,而这对于准确解释表现出高个体差异的复杂解剖结构至关重要。没有知识指导的特征提取,模型难以捕捉深层次且有意义的结构关系,从而影响形态学特征的可解释性。运动学-形态学融合的局限:Qi等(2024)在反向扩散过程的条件输入阶段通过简单的特征堆叠来整合运动学和形态学信息。然而,由于这种基本的融合技术没有考虑运动学和形态学数据之间不同的功能作用和时空差异,关键的条件细节可能会丢失。这降低了模型精确定位心肌瘢痕以及在不同患者解剖结构间泛化的能力。 本文提出了一种新颖的知识驱动的解释性条件扩散模型(K-ICDM),用于准确合成无对比剂的MIE图像。我们的K-ICDM引入了三个创新组件,以解决现有知识与数据驱动方法的局限性。心脏因果干预和知识驱动的认知整合策略分别旨在增强运动学和形态学模块中特征学习的可解释性,然后特定信息自适应融合策略加强运动学和形态学特征之间的相互作用,并将它们专门整合到用于合成MIE图像的反向扩散过程中。具体而言,心脏因果干预建立了异常心脏运动与心肌瘢痕之间的明确关系,揭示了心肌异常的真正潜在原因。它涉及通过修改特定的运动特征来生成反事实心肌应变场景,使我们能够观察这些变化如何影响模型的预测。通过隔离每次修改的影响,该方法消除了无关因素,为模型提供了清晰的因果可解释性,并能更精确地表征心肌异常。知识驱动的认知整合策略不仅在心脏信号拓扑知识的指导下对T1序列图像进行图像级整合,还基于这些图像之间的相关性在特征级学习形态学信息。它能够明确捕捉那些可解释的机械特征,以更准确地建模特征与心脏结构细节之间的关系,从而合成更清晰的边界。特定信息自适应融合策略根据运动学和形态学信息的特定贡献对它们进行融合。它首先将形态学信息编码为锚点特征到扩散主干中,以提供心脏基本结构的初始表征。随后,运动学信息作为语义特征被整合进来,并与已经包含形态学信息的扩散主干中的深层特征动态相互作用,使模型能够准确勾勒出心肌瘢痕。这种机制不仅允许运动学和形态学信息相互完善,还能保留更多细节信息。 1.1 贡献 总之,这项工作的主要贡献包括: - 一种新的无对比剂心肌梗死增强合成方法,消除了对比剂相关的健康风险,降低了医疗成本,并简化了临床工作流程。 - 一种新的知识与数据驱动框架,整合心脏生理学的先验知识,以指导模型提取可解释的机械特征。 - 一种新的基于扩散的跨序列融合策略,明确建模可解释的机械特征与输出图像之间的关系,从而实现更准确的瘢痕边界合成。 本工作在我们发表于MICCAI-2024的初步工作(Qi等,2024)的基础上,引入了几项新贡献:(1)我们引入了以心肌应变为变量的反事实干预,帮助模型识别心肌异常信息的真正潜在原因,从而为运动学提供清晰的因果可解释性。(2)我们创新性地引入心脏信号拓扑知识作为形态学学习的指导,对T1序列图像进行图像级和特征级的整合,从而为形态捕捉提供可解释性。(3)我们增强了运动学和形态学信息之间的深度相互作用,并根据它们的特定贡献将这两种信息编码为不同的条件输入到扩散主干中,从而保留更多细节信息。(4)我们在更大的数据集上进行了更全面的统计验证,本文对此进行了详细讨论。
Abatract
摘要
Synthesis of myocardial infarction enhancement (MIE) images without contrast agents (CAs) has shown greatpotential to advance myocardial infarction (MI) diagnosis and treatment. It provides results comparable tolate gadolinium enhancement (LGE) images, thereby reducing the risks associated with CAs and streamliningclinical workflows. The existing knowledge-and-data-driven approach has made progress in addressing thecomplex challenges of synthesizing MIE images (i.e., invisible myocardial scars and high inter-individualvariability) but still has limitations in the interpretability of kinematic inference, morphological knowledgeintegration, and kinematic-morphological fusion, thereby reducing the transparency and reliability of the modeland causing information loss during synthesis. In this paper, we proposed a knowledge-driven interpretativeconditional diffusion model (K-ICDM), which learns kinematic and morphological information from nonenhanced cardiac MR images (CINE sequence and T1 sequence) guided by cardiac knowledge, enablingthe synthesis of MIE images. Importantly, our K-ICDM introduces three key innovations that address theselimitations, thereby providing interpretability and improving synthesis quality. (1) A novel cardiac causalintervention that generates counterfactual strain to intervene in the inference process from motion maps toabnormal myocardial information, thereby establishing an explicit relationship and providing the clear causalinterpretability. (2) A knowledge-driven cognitive combination strategy that utilizes cardiac signal topologyknowledge to analyze T1 signal variations, enabling the model to understand how to learn morphologicalfeatures, thus providing interpretability for morphology capture. (3) An information-specific adaptive fusionstrategy that integrates kinematic and morphological information into the conditioning input of the diffusionmodel based on their specific contributions and adaptively learns their interactions, thereby preserving moredetailed information. Experiments on a broad MI dataset with 315 patients show that our K-ICDM achievesstate-of-the-art performance in contrast-free MIE image synthesis, improving structural similarity index measure(SSIM) by at least 2.1% over recent methods. These results demonstrate that our method effectively overcomesthe limitations of existing methods in capturing the complex relationship between myocardial motion and scardistribution and integrating of static and dynamic sequences, thus enabling the accurate synthesis of subtlescar boundaries.
无对比剂的心肌梗死增强(MIE)图像合成在推动心肌梗死(MI)诊断与治疗方面展现出巨大潜力。这种技术能生成与钆延迟增强(LGE)图像相当的结果,从而降低对比剂(CAs)相关风险,并简化临床工作流程。 现有的知识与数据驱动方法在应对MIE图像合成的复杂挑战(如心肌瘢痕不可见性和高个体间差异性)方面取得了一定进展,但在运动学推理的可解释性、形态学知识整合以及运动学-形态学融合等方面仍存在局限,这不仅降低了模型的透明度和可靠性,还会导致合成过程中的信息丢失。 本文提出了一种基于知识驱动的解释性条件扩散模型(K-ICDM),该模型在心脏知识的指导下,从非增强心脏磁共振图像(CINE序列和T1序列)中学习运动学和形态学信息,实现MIE图像的合成。重要的是,我们的K-ICDM引入了三项关键创新来解决这些局限,从而提升可解释性并改善合成质量: 1. 一种新颖的心脏因果干预机制,通过生成反事实应变来干预从运动图到心肌异常信息的推理过程,进而建立明确的关联关系,并提供清晰的因果可解释性。 2. 一种基于知识驱动的认知整合策略,利用心脏信号拓扑知识分析T1信号变化,使模型能够理解如何学习形态学特征,从而为形态捕捉提供可解释性。 3. 一种特定信息自适应融合策略,根据运动学和形态学信息的特定贡献,将其整合到扩散模型的条件输入中,并自适应学习它们之间的相互作用,从而保留更多细节信息。 在包含315名患者的大规模心肌梗死数据集上进行的实验表明,我们的K-ICDM在无对比剂MIE图像合成任务中达到了最先进的性能,与最新方法相比,结构相似性指数(SSIM)至少提高了2.1%。这些结果证明,我们的方法有效克服了现有方法在捕捉心肌运动与瘢痕分布之间复杂关系以及整合静态和动态序列方面的局限,能够准确合成细微的瘢痕边界。
Method
方法
Our K-ICDM (Fig. 4) takes non-enhanced CINE and T1 sequences,combining cardiac mechanics and signal topology knowledge as inputto synergistically drive the model in learning motion abnormalitiesand cardiac structural features, while interactively integrating thesefeatures into the reverse diffusion process for interpretable and accurateMIE image synthesis. It is composed of the Cardiac Mechanics-GuidedKinematics Intervention (CM-GKI) module, the Signal Topology-PriorMorphological Interpretation (ST-PMI) module, and the TargetedIntegration-Adaptive Interaction Diffusion (TI-AID) model. CM-GKIdesigns a causal intervention network based on myocardial straingenerating counterfactual strain to intervene in abnormal myocardialprediction, thereby establishing the explicit causal relationship betweenabnormal cardiac motion and scars. ST-PMI introduces an T1 signal calculation pattern based on cardiac signal topology knowledge to guidethe weighted integration of morphological features, thereby enablingthe model to understand the spatial relationships of cardiac tissues. TIAID designs an anchor and semantic conditioning network to facilitatethe collaborative synthesis of cardiac structures and myocardial scars,thereby preserving the detailed information for accurate synthesis
我们提出的 K-ICDM 模型(图 4)以非增强 CINE 序列和 T1 序列为输入,结合心脏力学知识和信号拓扑知识,协同驱动模型学习运动异常和心脏结构特征,同时将这些特征交互式地整合到反向扩散过程中,实现可解释且准确的 MIE 图像合成。该模型由三个部分组成:心脏力学引导的运动学干预(CM-GKI)模块、信号拓扑先验的形态学解释(ST-PMI)模块,以及靶向整合 - 自适应交互扩散(TI-AID)模型。CM-GKI 模块:基于心肌应变设计因果干预网络,通过生成反事实应变来干预心肌异常预测,从而建立异常心脏运动与瘢痕之间的明确因果关系。ST-PMI 模块:引入基于心脏信号拓扑知识的 T1 信号计算模式,指导形态学特征的加权整合,使模型能够理解心脏组织的空间关系。TI-AID 模型:设计锚点与语义条件网络,促进心脏结构与心肌瘢痕的协同合成,从而保留细节信息以实现精准合成。
Conclusion
结论
In this paper, we propose a novel knowledge-driven interpretativeconditional diffusion model for directly synthesizing enhanced cardiac images from non-enhanced cardiac MR images. The approachproposes an innovative cardiac causal intervention to establish anexplicit relationship between abnormal cardiac motion and myocardialscars, thereby providing the causal interpretability of myocardial scarprediction. Additionally, the approach proposes a new knowledgedriven cognitive combination strategy, which introduces cardiac signaltopology knowledge to guide the model in learning cardiac morphological information from T1 signal variations, thereby providing theinterpretability of morphological feature capture. Finally, the approachproposes a information-specific adaptive fusion strategy, which adaptively fuses kinematic and morphological information based on theirspecific contributions into the diffusion backbone, thus preserving moredetailed information. The proposed method achieved a new state-ofthe-art performance in myocardial infarction enhancement synthesis,with an SSIM of 0.803, a PSNR of 27.62, an LPIPS of 0.078, and anNMSE of 0.064, using data from 315 subjects. These results indicatethat the proposed approach has great potential to serve as a reliableand safe alternative for cardiac MR image enhancement, potentiallyeliminating the need for contrast agents, reducing medical costs, andsimplifying clinical workflows. However, there are still some limitations in the current research. Image data in clinical environments areoften affected by patient movement and equipment differences, whichimpacts the model’s performance. Therefore, future research couldexplore methods to address motion artifacts and data heterogeneity.
本文提出了一种新颖的知识驱动的可解释条件扩散模型,用于直接从非增强心脏磁共振图像中合成增强心脏图像。该方法创新性地提出了心脏因果干预,以建立异常心脏运动与心肌瘢痕之间的明确关系,从而为心肌瘢痕预测提供因果可解释性。此外,该方法还提出了一种新的知识驱动认知整合策略,引入心脏信号拓扑知识,指导模型从T1信号变化中学习心脏形态学信息,进而为形态学特征捕捉提供可解释性。最后,该方法提出了特定信息自适应融合策略,基于运动学信息和形态学信息的特定贡献,将它们自适应地融合到扩散主干中,从而保留更多细节信息。 所提出的方法在心肌梗死增强合成方面达到了新的最先进性能,利用315名受试者的数据,取得了0.803的结构相似性指数(SSIM)、27.62的峰值信噪比(PSNR)、0.078的学习感知图像patch相似性(LPIPS)和0.064的归一化均方误差(NMSE)。这些结果表明,所提出的方法有很大潜力成为一种可靠且安全的心脏磁共振图像增强替代方案,有可能无需使用对比剂、降低医疗成本并简化临床工作流程。 然而,当前研究仍存在一些局限性。临床环境中的图像数据常受患者运动和设备差异的影响,这会对模型性能产生影响。因此,未来的研究可以探索解决运动伪影和数据异质性的方法。
Figure
图
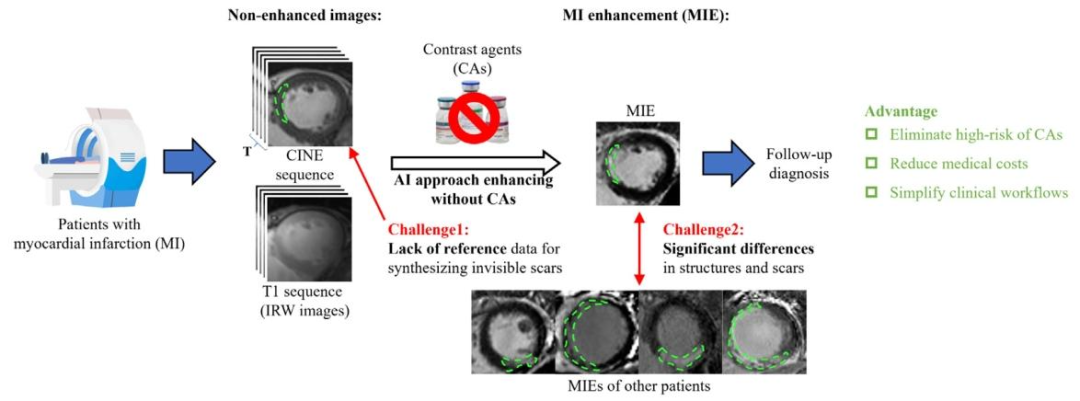
Fig. 1. Contrast-free myocardial infarction enhancement (MIE) technique holds great potential as a reliable and safe method for cardiac magnetic resonance (MR) imageenhancement. However, synthesizing MIE images without CAs is a significant challenge due to the invisibility of myocardial scars on CINE sequences and the high variabilityamong individuals, which together result in a lack of reference for synthesizing myocardial scars and significant differences in cardiac structures and scar location
图 1. 无对比剂心肌梗死增强(MIE)技术作为一种可靠且安全的心脏磁共振(MR)图像增强方法具有巨大潜力。然而,由于心肌瘢痕在 CINE 序列上不可见,且个体间差异较大,无对比剂合成 MIE 图像面临重大挑战 —— 这两点共同导致合成心肌瘢痕时缺乏参考依据,同时心脏结构和瘢痕位置也存在显著差异
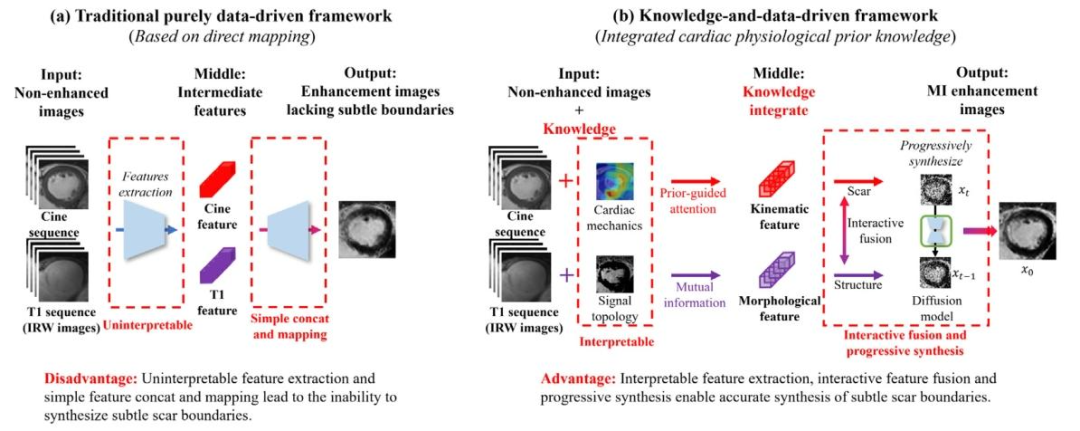
Fig. 2. Comparison between the traditional purely data-driven framework (left) and our proposed knowledge-and-data-driven framework (right).
图 2. 传统纯数据驱动框架(左)与我们提出的知识 - 数据驱动框架(右)的对比
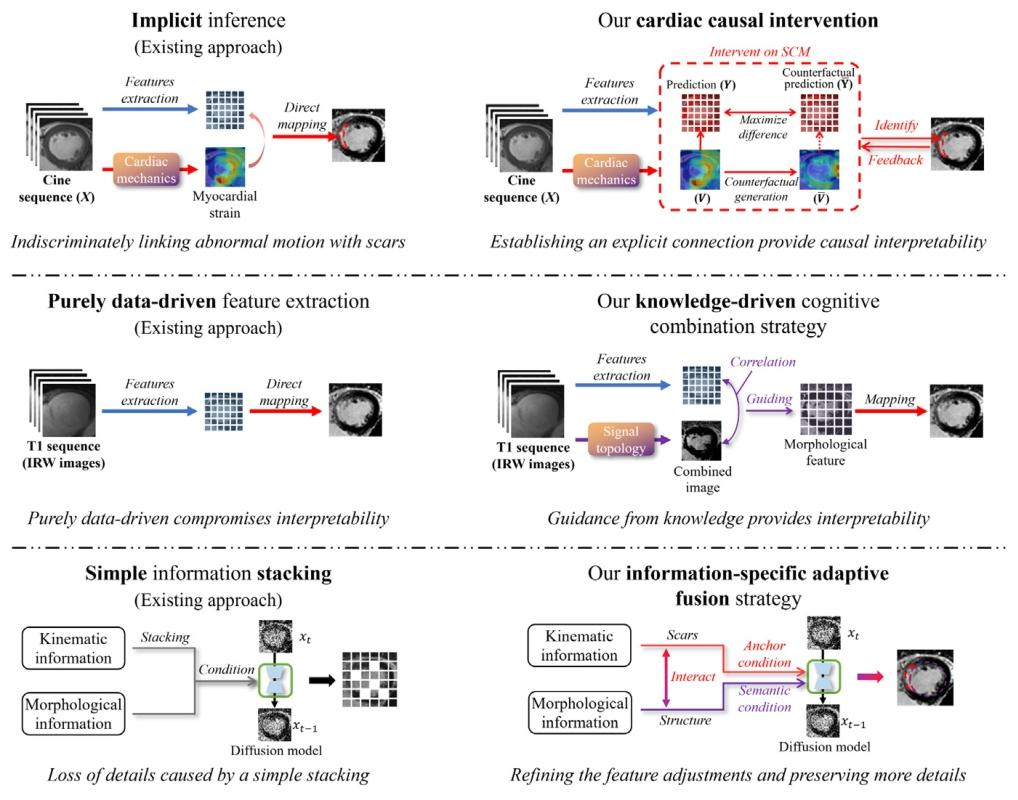
Fig. 3. Improvements in the interpretability of kinematic inference, morphological knowledge integration, and kinematic-morphological fusion in our proposed method comparedto the existing approach.
图 3. 与现有方法相比,我们提出的方法在运动学推理的可解释性、形态学知识整合以及运动学 - 形态学融合方面的改进
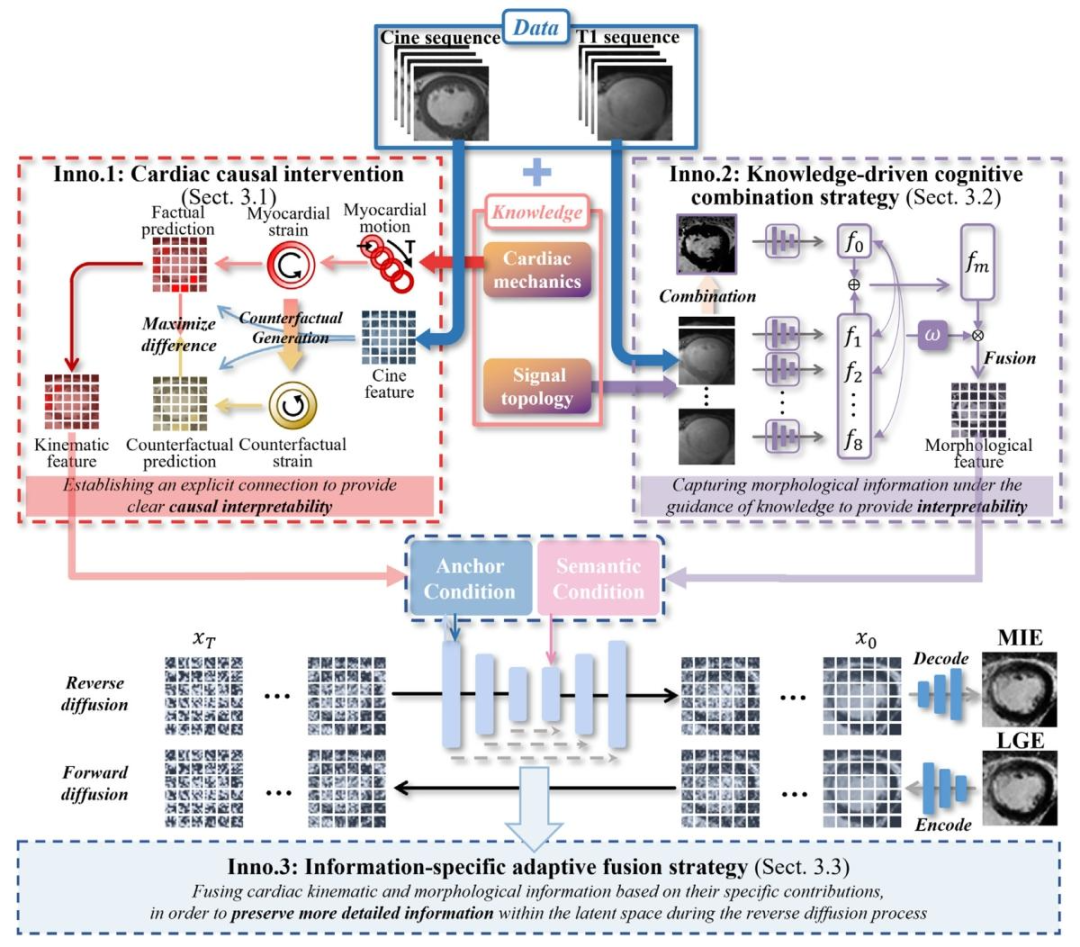
Fig. 4. Our K-ICDM approach incorporates cardiac knowledge (Cardiac mechanics and Signal topology) to learn kinematic information from CINE sequences and morphologicalinformation from T1 sequences. These types of information are then fused using a latent diffusion model to synthesize MIE images. Our approach offers three novel contributionsin the areas of the interpretability of kinematic inference (cardiac causal intervention), morphological knowledge integration (knowledge-driven cognitive combination strategy)and kinematic-morphological fusion (information-specific adaptive fusion strategy) to provide interpretability and synthesis quality
图4. 我们的K-ICDM方法整合心脏知识(心脏力学和信号拓扑),从CINE序列中学习运动学信息,从T1序列中学习形态学信息。然后使用潜在扩散模型融合这些信息以合成MIE图像。我们的方法在运动学推理的可解释性(心脏因果干预)、形态学知识整合(知识驱动的认知整合策略)和运动学-形态学融合(特定信息自适应融合策略)方面提供了三项新颖贡献,以提升可解释性和合成质量。
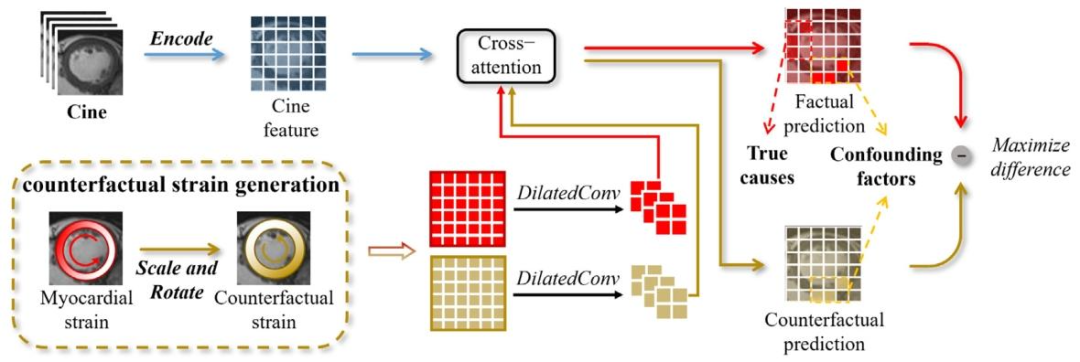
Fig. 5. The proposed CM-GKI utilizes counterfactual strain maps generated from real myocardial strain maps to intervene in abnormal myocardium prediction, thereby establishingcausal links between abnormal myocardium and their true underlying causes, thus providing explicit causal interpretability
图 5. 所提出的 CM-GKI 模块利用从真实心肌应变图生成的反事实应变图,干预心肌异常预测,从而建立心肌异常与其真正潜在原因之间的因果关联,进而提供明确的因果可解释性
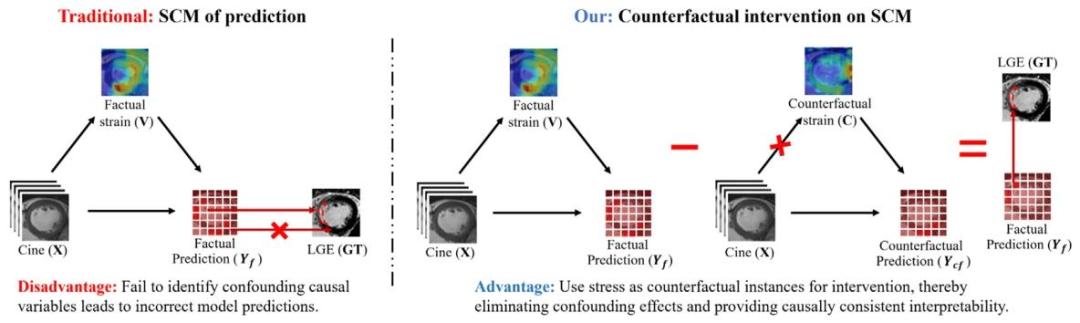
Fig. 6. Our counterfactual intervention innovatively introduces myocardial strain as counterfactual instances to eliminate confounders in the causal model, enhancing the model’sprediction of abnormal myocardium and providing causally consistent interpretability
图6. 我们的反事实干预创新性地引入心肌应变作为反事实实例,以消除因果模型中的混杂因素,增强模型对心肌异常的预测,并提供因果一致性的可解释性。
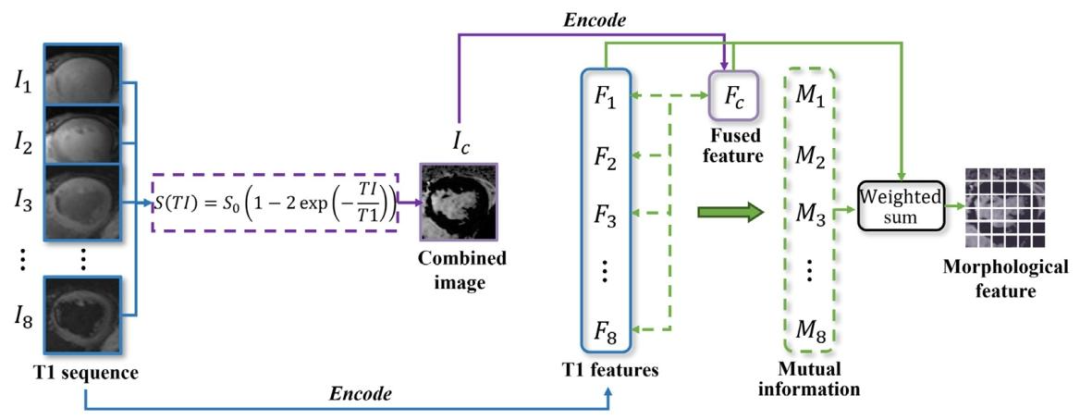
Fig. 7. The proposed ST-PMI integrates T1 sequence morphological information at the image level by analyzing T1 signal variations, and extracts detailed morphological featuresat the feature level based on the mutual information, thereby providing the interpretability of myocardial structure capture.
图7. 所提出的ST-PMI通过分析T1信号变化在图像级整合T1序列的形态学信息,并基于互信息在特征级提取详细的形态学特征,从而为心肌结构捕捉提供可解释性。
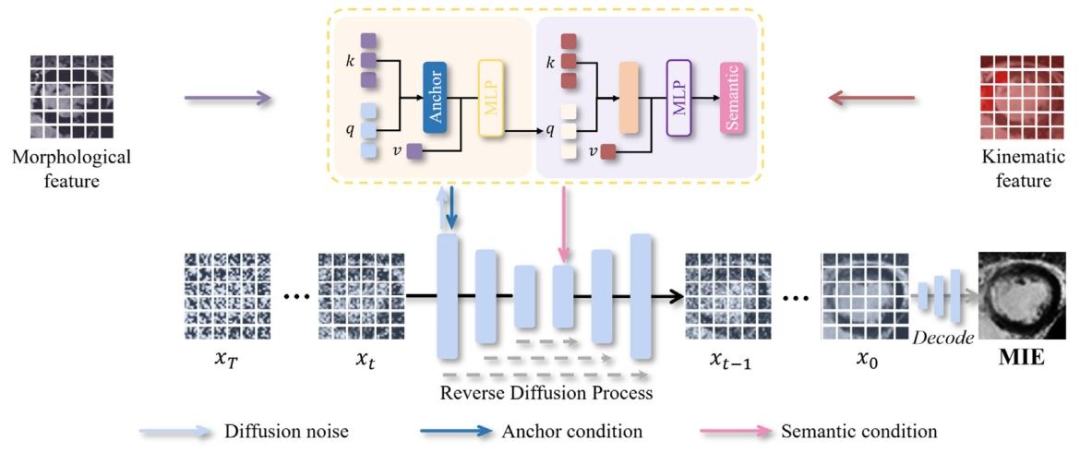
Fig. 8. The proposed TI-AID designs an anchor and semantic conditioning network that integrates features into the reverse diffusion process based on their unique contributionsand perform adaptive interactions for mutual enhancement.
图8. 所提出的TI-AID设计了锚点与语义条件网络,该网络根据特征的独特贡献将其整合到反向扩散过程中,并进行自适应交互以实现相互增强。
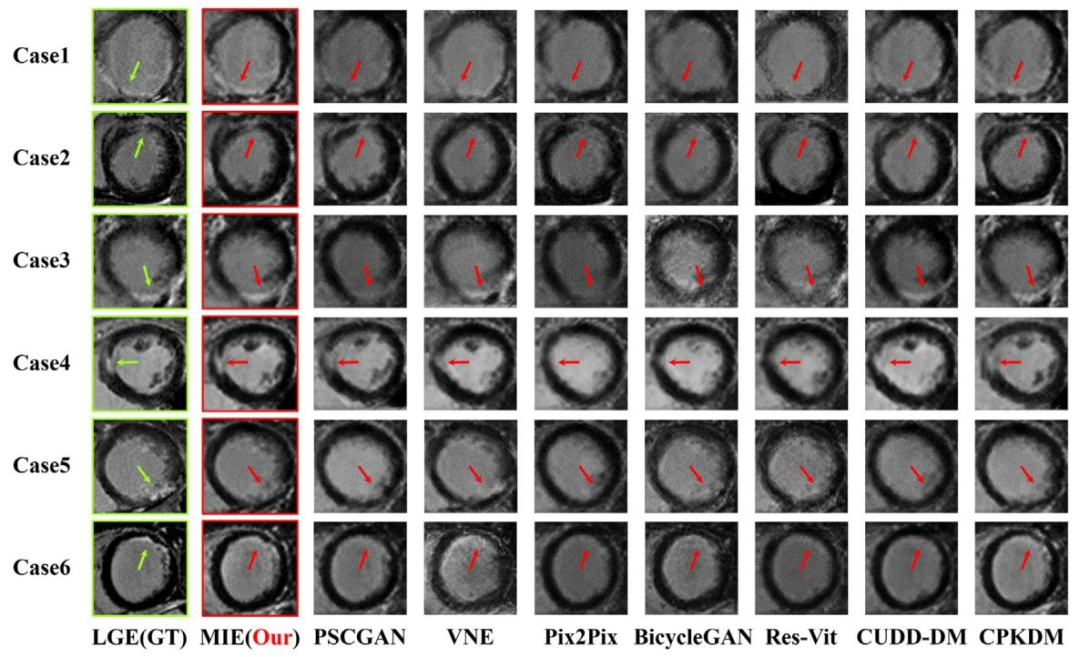
Fig. 9. K-ICDM has demonstrated accurate synthesis of enhanced images from non-enhanced cardiac MR images. Our approach has outperformed all seven existing state-of-the-artmethods.
图9. K-ICDM已证明能够从非增强心脏磁共振图像中准确合成增强图像。我们的方法优于所有七种现有的最先进方法。
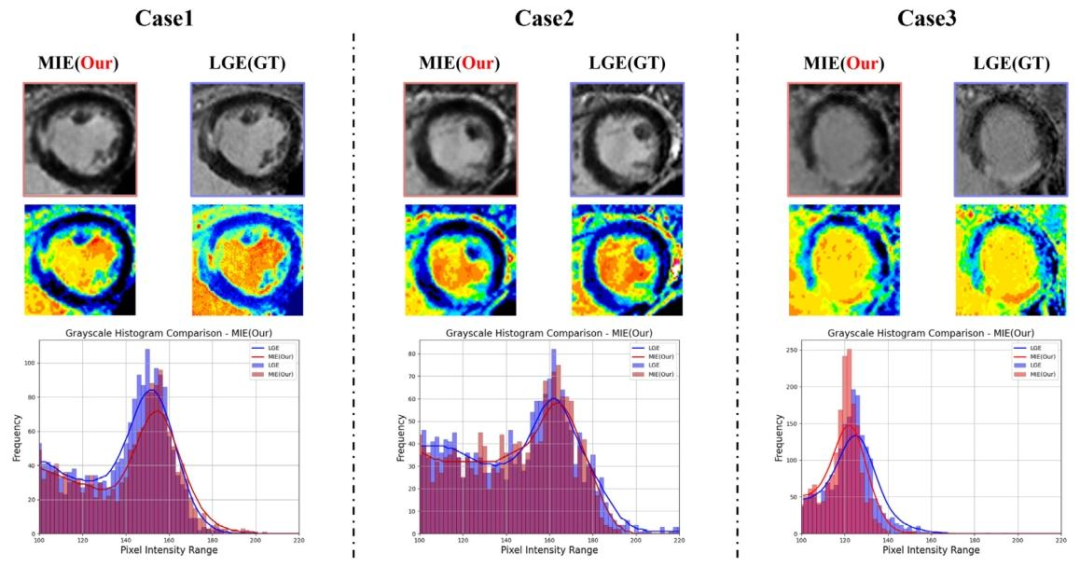
Fig. 10. Grayscale histograms compare the pixel intensity distributions of the LGE image with the MIE image (our K-ICDM). The histogram of the MIE image shows a pixelintensity distribution highly similar to that of the LGE image, indicating that our K-ICDM demonstrates superior performance in contrast-free MI image enhancement. The leftpanel provides a visual comparison between the MIE and LGE images using a heat map. The right panel shows the pixel intensity distributions of the MIE and LGE images, withthe curves smoothed using Gaussian filtering
图10. 灰度直方图对比了LGE图像与MIE图像(我们的K-ICDM)的像素强度分布。MIE图像的直方图显示出与LGE图像高度相似的像素强度分布,表明我们的K-ICDM在无对比剂心肌梗死图像增强方面表现出优异性能。左图通过热图直观对比了MIE图像与LGE图像;右图展示了MIE图像和LGE图像的像素强度分布,曲线经高斯滤波平滑处理。
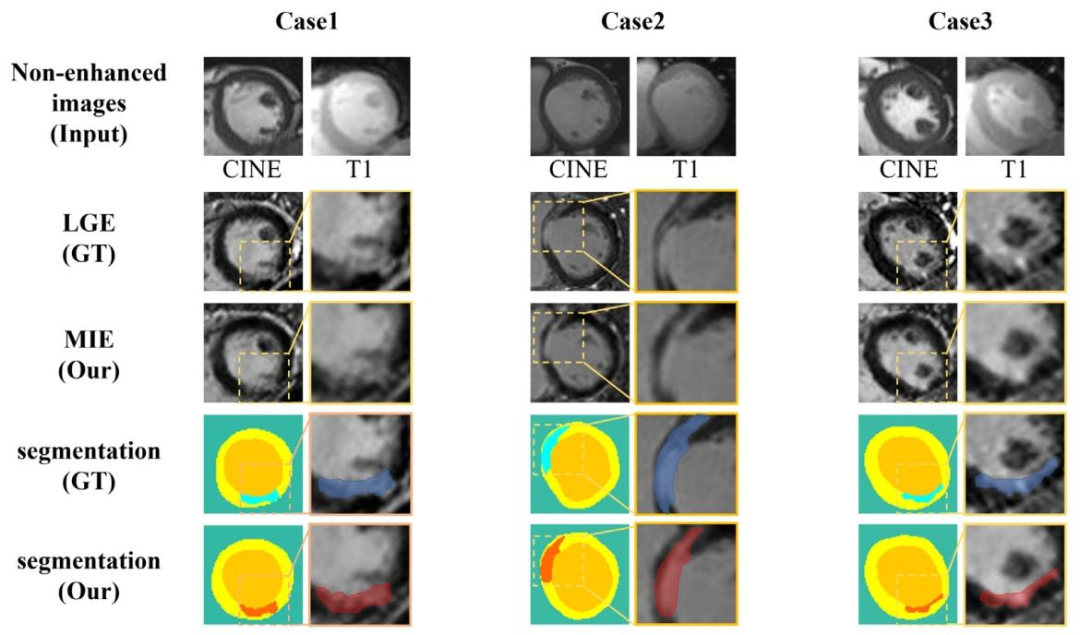
Fig. 11. Our K-ICDM synthesized high-quality MIE images without CAs, showing high consistency with LGE images in terms of myocardial structure and scar location. Notably,myocardial scar regions are invisible in non-enhanced images. Following manual tissue segmentation by expert physicians, the scars (MIE: red , LGE: blue ), healthymyocardium (yellow ), and blood pool (orange ) segmented from our MIE images exhibit high consistency with the segmentation results of the LGE images (ground truth)in terms of shape, location, and size
图11. 我们的K-ICDM在无对比剂情况下合成了高质量的MIE图像,在心肌结构和瘢痕位置方面与LGE图像高度一致。值得注意的是,心肌瘢痕区域在非增强图像中不可见。经过专家医师的手动组织分割后,从我们的MIE图像中分割出的瘢痕(MIE:红色 ,LGE:蓝色 )、健康心肌(黄色 )和血池(橙色 ),在形状、位置和大小上与LGE图像(真值)的分割结果高度一致。
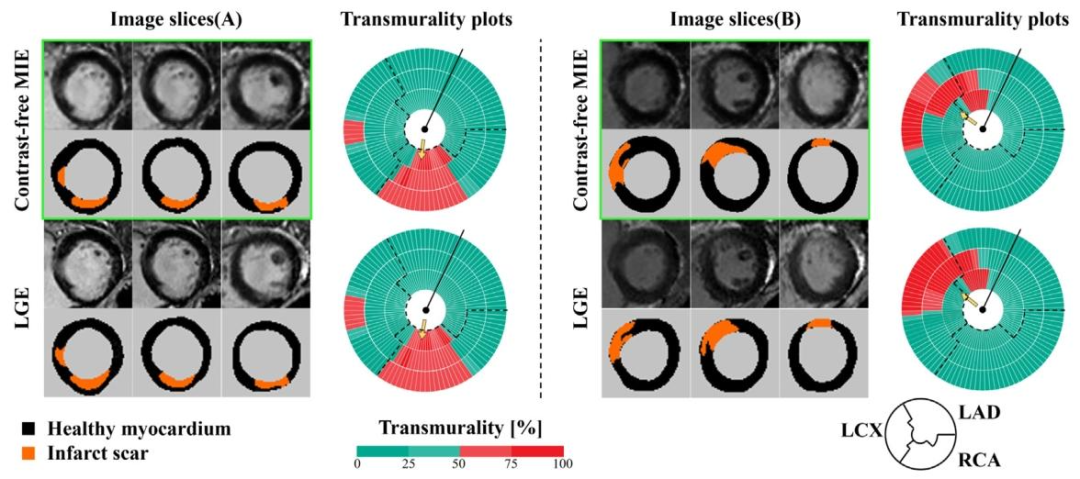
Fig. 12. MIE revealed a right coronary artery (RCA) territory myocardial scar in patient A, and myocardial scars in both the left circumflex artery (LCX) and left anterior descendingartery (LAD) territories in patient B, all with MIE images closely matching the signals revealed by LGE. The left panel provides a visual comparison of MIE and LGE images usingthree short-axis slices from the same patient, with scar areas marked in orange. The right panel displays the scar transmurality measured by MIE and LGE, indicating the likelihoodof myocardial viability (0 to 25%, viable; 26% to 50%, likely viable; 51% to 75%, likely nonviable; 76% to 100%, nonviable). Dashed lines delineate the presumed boundariesbetween myocardial regions (LAD, left anterior descending artery, LCx, left circumflex artery, and RCA, right coronary artery.), and arrows point to the scar areas.
图12. MIE显示患者A存在右冠状动脉(RCA)供血区域的心肌瘢痕,患者B存在左回旋支(LCX)和左前降支(LAD)供血区域的心肌瘢痕,所有MIE图像均与LGE显示的信号高度匹配。左图使用同一患者的三个短轴切面直观对比MIE和LGE图像,瘢痕区域以橙色标记。右图展示MIE和LGE测量的瘢痕透壁性,透壁性可提示心肌活力(0-25%为有活力;26%-50%为可能有活力;51%-75%为可能无活力;76%-100%为无活力)。虚线勾勒出心肌各区域(LAD:左前降支、LCX:左回旋支、RCA:右冠状动脉)的推测边界,箭头指向瘢痕区域。
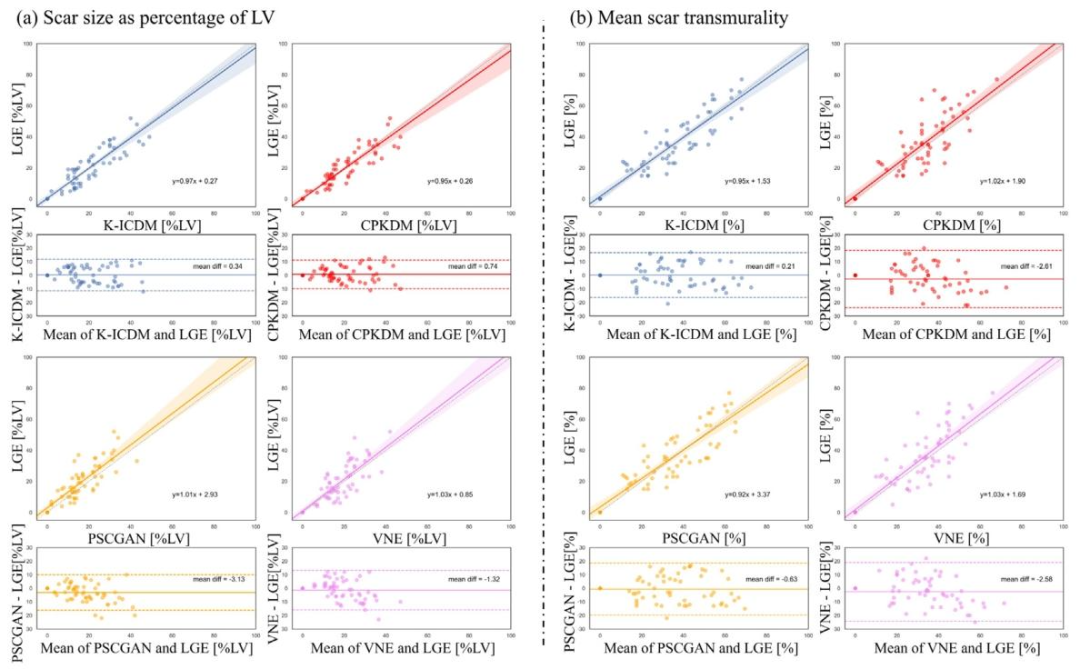
Fig. 13. Comparison of myocardial scar and transmurality analysis between images synthesized by the K-ICDM and other methods. (a) Scar size expressed as a volume fraction ofthe sampled left ventricular (LV) myocardium; (b) Mean transmurality of scarred chords per patient, evaluated via Bland–Altman analysis. For the 55 test patients with myocardialscar, mean scar transmurality was calculated by averaging the transmural scar extent of all chords that exhibited at least 1% scar extent on all available slices, using the centerlinechord method. In addition, 10 normal controls (with no detectable myocardial scar on LGE images) were included for comparison; for these controls, both scar volume fractionand transmurality were 0%.
图13. K-ICDM合成图像与其他方法合成图像在心肌瘢痕及透壁性分析上的对比。(a)瘢痕大小以采样的左心室(LV)心肌的体积分数表示;(b)通过Bland-Altman分析评估每位患者瘢痕区域的平均透壁性。对于55名存在心肌瘢痕的测试患者,采用中线弦法,对所有可用切面上瘢痕程度至少为1%的区域,取其透壁瘢痕程度的平均值,作为平均瘢痕透壁性。此外,纳入10名正常对照(LGE图像中未检测到心肌瘢痕)进行对比;这些对照的瘢痕体积分数和透壁性均为0%。
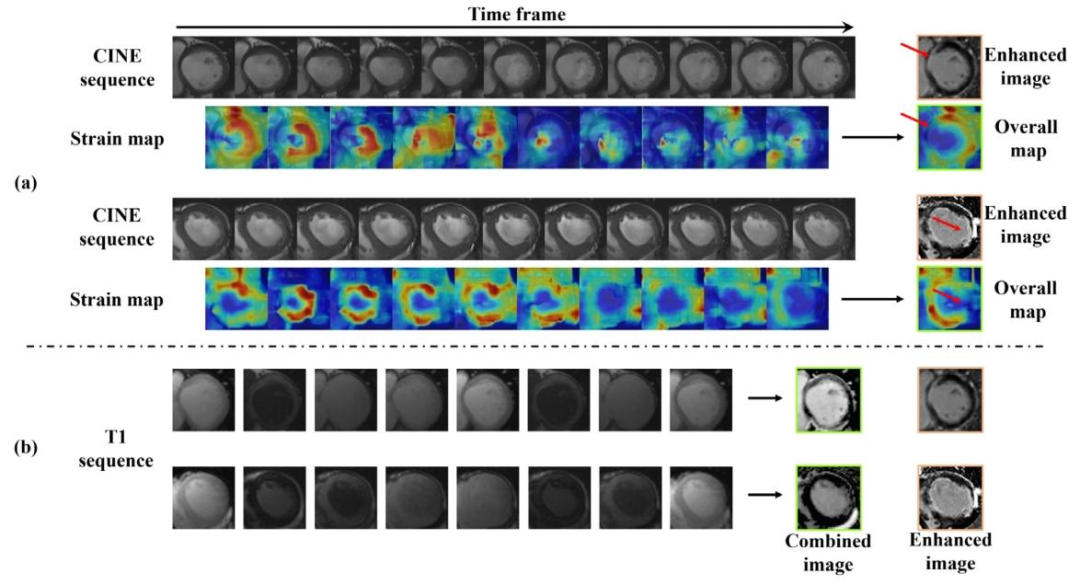
Fig. 14. Our K-ICDM provides the interpretability of contrast-free MIE synthesis. (a) Strain maps between adjacent frames in the CINE sequence, followed by an overall strainmap, highlighting the hypokinetic regions within the myocardium. (b) Combined signal image of T1 sequence, revealing clear cardiac structural boundaries
图14. 我们的K-ICDM为无对比剂MIE合成提供了可解释性。(a)CINE序列中相邻帧之间的应变图,以及整体应变图,突出显示了心肌内的运动减弱区域。(b)T1序列的联合信号图像,显示出清晰的心脏结构边界。
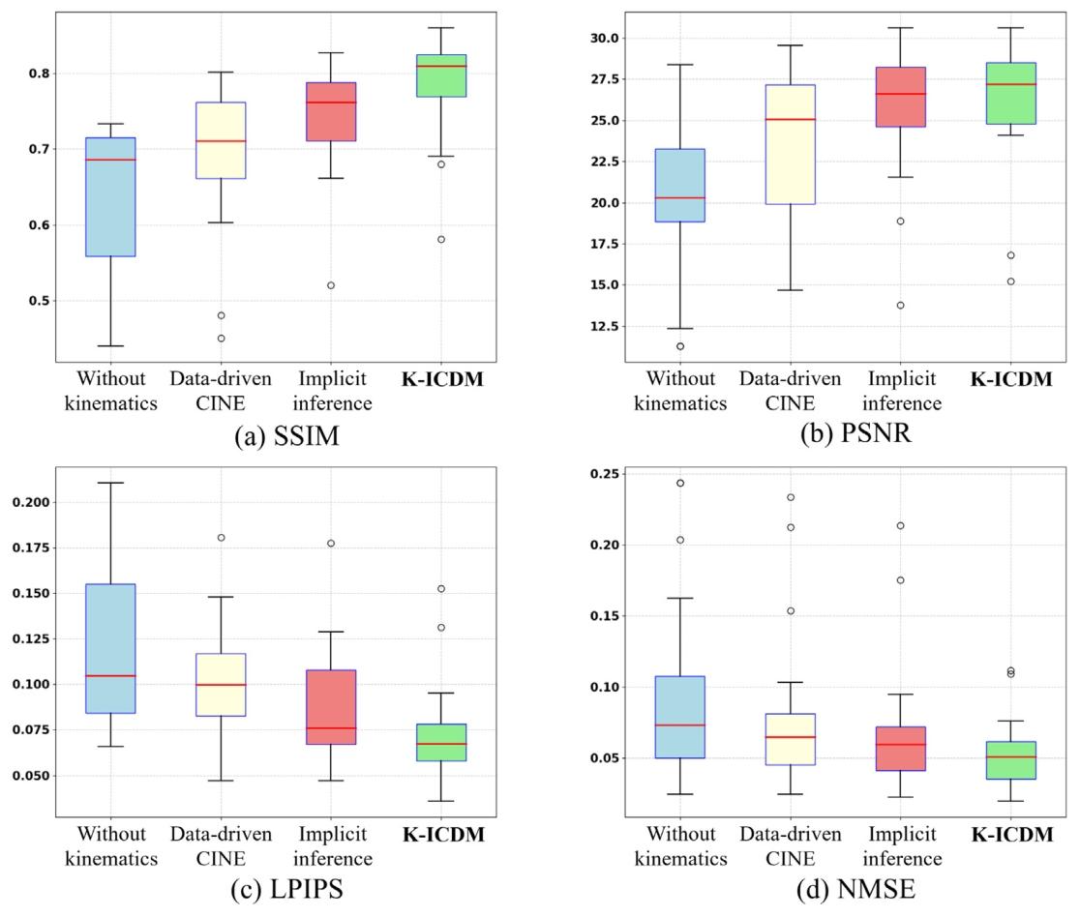
Fig. 15. K-ICDM’s cardiac causal intervention was able to capture the true underlying causes of myocardial abnormalities, resulting in superior synthesis performance comparedto various ablation versions, as demonstrated by metrics such as SSIM, PSNR, LPIPS, and NMSE.
图15. K-ICDM的心脏因果干预能够捕捉到心肌异常的真正潜在原因,与各种消融版本相比,在结构相似性指数(SSIM)、峰值信噪比(PSNR)、学习感知图像patch相似性(LPIPS)和归一化均方误差(NMSE)等指标上均表现出更优异的合成性能。
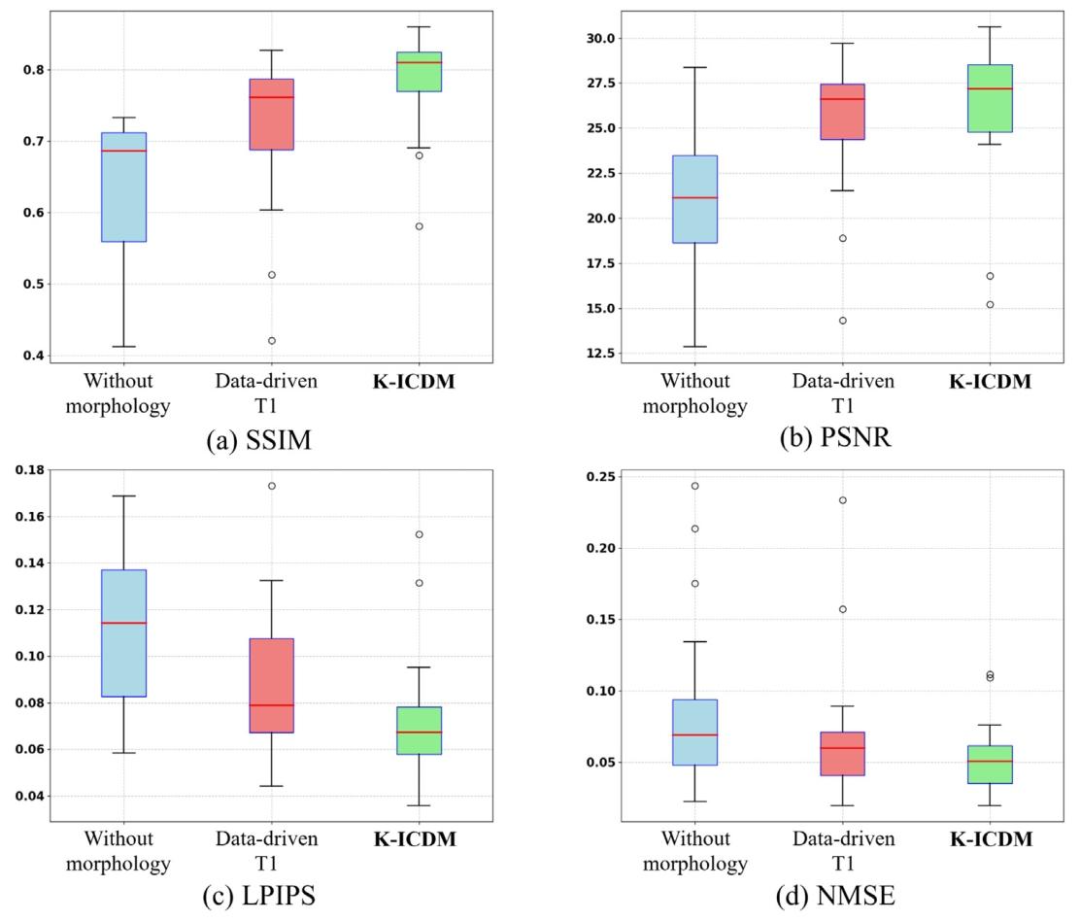
Fig. 16. K-ICDM’s knowledge-driven cognitive combination strategy was able to better guide the model in capturing and inferring cardiac morphological information, resulting insuperior synthesis performance compared to various ablation versions, as validated by metrics such as SSIM, PSNR, LPIPS, and NMSE.
图16. K-ICDM的知识驱动认知整合策略能够更好地指导模型捕捉和推断心脏形态学信息,与各种消融版本相比,在结构相似性指数(SSIM)、峰值信噪比(PSNR)、学习感知图像patch相似性(LPIPS)和归一化均方误差(NMSE)等指标上均验证了其更优异的合成性能。

Fig. 17. K-ICDM’s information-specific adaptive fusion strategy enhances the dynamic interaction capability between kinematic and morphological information. Our approachoutperformed various ablation versions in key metrics such as SSIM, PSNR, LPIPS, and NMSE, thereby demonstrating its effectiveness in MIE image synthesis.
图17. K-ICDM的特定信息自适应融合策略增强了运动学信息与形态学信息之间的动态交互能力。我们的方法在结构相似性指数(SSIM)、峰值信噪比(PSNR)、学习感知图像patch相似性(LPIPS)和归一化均方误差(NMSE)等关键指标上优于各种消融版本,从而证明了其在MIE图像合成中的有效性。
Table
表
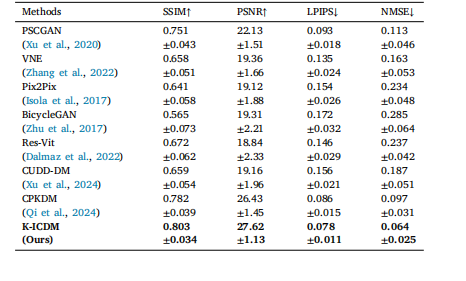
Table 1Our K-ICDM achieves a new state-of-the-art performance in the field of contrast-freeMIE image synthesis compared to all seven comparative methods at the image level.
表1 在图像级别上,与所有七种对比方法相比,我们的K-ICDM在无对比剂MIE图像合成领域达到了新的最先进性能。
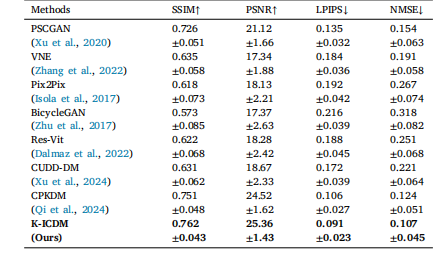
Table 2Our K-ICDM compared with other methods at the region level
表2 在区域级别上,我们的K-ICDM与其他方法的对比。
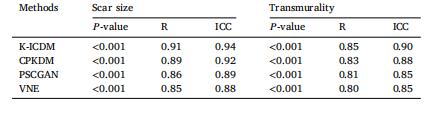
Table 3Comparison of correlation coefficients between synthesized MIE images and LGE imagesusing the K-ICDM and other methods. Scar size is expressed as a volume fraction of thesampled left ventricular (LV) myocardium, and mean scar transmurality is quantifiedper patient. Data are based on 65 test patients, including 10 normal controls. The 𝑃 -value represents the statistical significance of the correlation, R represents the Pearsoncorrelation coefficient, and ICC represents the intraclass correlation coefficient.
表3 K-ICDM与其他方法的合成MIE图像和LGE图像之间的相关系数对比 瘢痕大小以采样的左心室(LV)心肌的体积分数表示,平均瘢痕透壁性按患者量化。数据基于65名测试患者,包括10名正常对照。𝑃值表示相关性的统计显著性,R表示皮尔逊相关系数,ICC表示组内相关系数。
